Vaso occlusive crisis (VOC) is the most common and debilitating clinical manifestation of sickle cell disease (SCD). It is responsible for a significant volume of hospitalizations and emergency department visits by patients suffering from this inherited blood disorder. Vaso Occlusive Crisis Drug Pipeline Analysis Recent studies suggest that nearly half of individuals with sickle cell disease experience vaso-occlusive crises, with some patients having six or more such episodes annually. These episodes occur when sickled red blood cells block blood flow, causing intense pain and potential organ damage. Repeated VOC episodes can lead to severe health complications, making it imperative to develop effective therapeutic interventions. Vaso Occlusive Crisis Drug Pipeline Analysis This heightened demand for better treatments has spurred an intense focus on the drug pipeline for VOC, aiming to offer relief and potentially prevent these painful episodes from occurring.
Vaso Occlusive Crisis Drug Pipeline Analysis, focusing on the latest developments, market trends, and key players. We will also address the impact of external factors, such as COVID-19, on drug development and market growth. Additionally, the drug pipeline dynamics, segmentation, and the role of innovative therapies will be discussed in detail.
Get a Free Sample Report with a Table of Contents: https://www.expertmarketresearch.com/clinical-trials/vaso-occlusive-crisis-drug-pipeline-analysis/requestsample
Vaso Occlusive Crisis Drug Pipeline Analysis Overview
Vaso occlusive crisis (VOC) is a significant concern in the management of sickle cell disease, which affects millions of people globally, with a higher prevalence in Africa, the Middle East, and certain parts of Asia. VOC episodes can lead to severe pain, organ dysfunction, and life-threatening complications, making it a priority for healthcare professionals to develop more effective treatments. Over the years, the pipeline for VOC therapies has evolved from pain management approaches to more targeted drugs that aim to reduce the frequency, severity, and duration of these crises.
The Vaso Occlusive Crisis drug pipeline comprises a diverse set of therapeutic candidates, including small molecules, biologics, gene therapies, and innovative pain management solutions. Pharmaceutical companies, biotechnology firms, and academic institutions have actively worked on developing drugs to address VOC's underlying pathophysiology. These efforts aim to improve patient outcomes by focusing not only on pain management but also on preventing the triggers of VOC, reducing the frequency of episodes, and minimizing the long-term health effects of the disease.
Read Full Report with Table of Contents: https://www.expertmarketresearch.com/clinical-trials/vaso-occlusive-crisis-drug-pipeline-analysis
Key Phases in the Vaso Occlusive Crisis Drug Pipeline:
- Preclinical Stage: Research and development in this stage involve the identification of potential drug candidates that can modify the pathophysiology of VOC and sickle cell disease.
- Clinical Trials: Once a drug shows promise, it enters clinical trials in Phases I, II, and III, testing safety, efficacy, and optimal dosing regimens.
- Marketed Products: Some drugs may already be on the market or in the late stages of development, offering new treatment options for SCD patients suffering from VOC.
Vaso Occlusive Crisis Drug Pipeline Analysis Dynamics
The dynamics of the Vaso Occlusive Crisis drug pipeline are influenced by several factors, ranging from the increasing global prevalence of sickle cell disease to advances in medical research and regulatory approval processes.
1. Growing Market Demand:
As mentioned earlier, VOC is a common manifestation of sickle cell disease, affecting millions of patients. In developed countries such as the United States and Europe, there has been a growing focus on the management of SCD, including the development of targeted therapies for VOC. The increasing demand for treatments to address VOC has driven pharmaceutical companies and research institutions to invest in novel drug candidates.
2. Advances in Understanding Sickle Cell Disease:
The underlying mechanisms of VOC are being better understood through advancements in genetics, molecular biology, and cellular research. By identifying the key drivers of VOC, researchers can develop drugs that target specific molecular pathways involved in the crisis, offering more effective and less invasive treatment options.
3. Targeted Therapies and Personalized Medicine:
The development of targeted therapies has gained significant traction in the treatment of VOC. With personalized medicine becoming increasingly prevalent, there is an emphasis on tailoring treatment based on individual patient characteristics, including genetic factors, disease severity, and co-existing conditions. This shift towards precision medicine offers a more focused approach to treating VOC.
External Vaso Occlusive Crisis Drug Pipeline Analysis Trends
Several external trends are influencing the drug development pipeline for VOC therapies. These include:
1. Gene Therapy and Stem Cell-Based Approaches:
Gene therapies are emerging as a potential cure for sickle cell disease, addressing the root cause of the condition rather than just managing its symptoms. Technologies like CRISPR/Cas9 gene editing have shown promise in clinical trials for treating SCD, which could also impact VOC management in the future. Stem cell-based therapies are also being explored as a potential option to replace sickle-shaped cells with healthy red blood cells, offering the possibility of long-term relief from VOC.
2. Regulatory Support and Fast-Track Approvals:
Regulatory agencies like the U.S. FDA and the European Medicines Agency (EMA) have provided fast-track designations and priority reviews for therapies targeting SCD and VOC. This support accelerates the development of new drugs and provides quicker access to treatment options for patients in need.
3. Focus on Non-Opioid Pain Management:
As opioid use becomes more scrutinized due to its addictive nature and potential for abuse, the need for non-opioid pain management options for VOC patients has grown. There is a rising focus on developing drugs that can provide effective pain relief without the risks associated with opioids.
Vaso Occlusive Crisis Drug Pipeline Analysis Segmentation
The Vaso Occlusive Crisis drug pipeline can be segmented based on drug type, stage of development, therapeutic approach, and treatment outcomes. This segmentation helps in understanding the landscape of VOC therapies and the ongoing developments in the field.
1. Drug Type:
- Small Molecule Drugs: These are conventional drugs that target specific pathways involved in VOC. Examples include hydroxyurea and other similar compounds.
- Biologics: Monoclonal antibodies and other biologics are being developed to target specific proteins involved in the pathogenesis of VOC.
- Gene Therapies: These therapies aim to address the root cause of SCD, potentially providing a cure for VOC.
- Pain Management Drugs: Non-opioid analgesics and other pain-relieving treatments are crucial for managing VOC episodes.
2. Stage of Development:
- Preclinical: Research into new drug candidates and treatment mechanisms.
- Clinical Trials (Phase I, II, III): Drugs undergoing rigorous testing for safety, efficacy, and optimal use.
- Approved Treatments: Drugs that have received regulatory approval and are available in the market.
Vaso Occlusive Crisis Drug Pipeline Analysis Growth
The growth of the Vaso Occlusive Crisis drug pipeline has been fueled by several factors, including advancements in medical research, a better understanding of the disease, and increased investment from both public and private sectors. According to market research, the global sickle cell disease therapeutics market is expected to grow significantly, with a substantial portion attributed to VOC treatment development.
Key growth drivers include:
- Increase in Sickle Cell Disease Prevalence: As more individuals are diagnosed with sickle cell disease globally, the demand for new and effective treatments for VOC will continue to grow.
- Technological Advancements: Progress in gene editing, cell therapies, and targeted drug development is driving the pipeline forward.
- Investment in Research and Development: Both large pharmaceutical companies and smaller biotech firms are investing heavily in the development of VOC treatments.
Recent Vaso Occlusive Crisis Drug Pipeline Analysis Market
In recent years, the VOC drug pipeline has seen significant advances, with several promising treatments entering clinical trials. Some of the most notable developments include:
- Voxelotor (Oxbryta): Approved by the FDA in 2019, voxelotor targets hemoglobin polymerization and improves oxygen delivery to tissues, reducing VOC frequency.
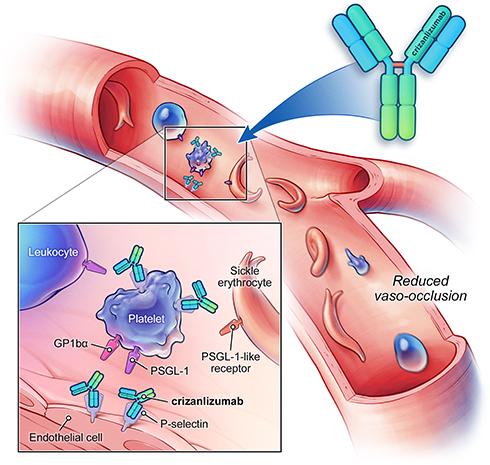
- Crizanlizumab (Adakveo): This monoclonal antibody targets P-selectin to reduce VOC frequency by preventing the adhesion of sickled red blood cells to the blood vessel walls.
- Gene Therapies: Ongoing trials are investigating gene therapy as a potential long-term solution to address VOC by curing sickle cell disease at its genetic root.
Vaso Occlusive Crisis Drug Pipeline Analysis Scope
The scope of the Vaso Occlusive Crisis drug pipeline is vast, encompassing a variety of treatment approaches aimed at improving the lives of individuals affected by sickle cell disease. With growing recognition of the challenges posed by VOC, the scope for new drug development continues to expand.
Vaso Occlusive Crisis Drug Pipeline Analysis Analysis
A thorough analysis of the pipeline reveals that significant progress has been made in the development of therapies that not only manage the pain associated with VOC but also target the underlying mechanisms of the disease. However, challenges remain in addressing the long-term management of VOC and reducing recurrence rates.
COVID-19 Impact Analysis
The COVID-19 pandemic has had a substantial impact on drug development across various therapeutic areas, including VOC. Delays in clinical trials, disrupted supply chains, and reallocation of healthcare resources have affected the progress of certain therapies. However, the pandemic has also spurred innovation, with virtual trials and remote patient monitoring becoming more prevalent in clinical development.
Key Players in the Vaso Occlusive Crisis Drug Pipeline
Several pharmaceutical and biotech companies are leading the charge in developing new treatments for Vaso Occlusive Crisis. Some of the key players include:
- AstraZeneca: Known for its research into novel therapies, including monoclonal antibodies and gene therapies for SCD and VOC.
- Novartis Pharmaceuticals: The maker of hydroxyurea and crizanlizumab, Novartis continues to develop innovative therapies for VOC management.
- Hoffmann-La Roche: Focused on immunotherapy and biologic treatments, Roche is involved in ongoing clinical trials targeting VOC.
FAQ
1. What is Vaso Occlusive Crisis?
Vaso occlusive crisis is a painful episode that occurs in patients with sickle cell disease when sickled red blood cells block blood flow, leading to pain and potential organ damage.
2. What are the treatment options for Vaso Occlusive Crisis?
Current treatments focus on pain management (e.g., opioids, non-opioid analgesics) and disease-modifying therapies (e.g., hydroxyurea, crizanlizumab).
3. How can gene therapy help in managing VOC?
Gene therapy aims to address the genetic root cause of sickle cell disease, potentially providing a long-term solution to VOC by restoring normal red blood cell function.
4. What are the latest trends in VOC drug development?
Gene therapies, monoclonal antibodies, and targeted small molecules are the key trends driving VOC drug development.
5. How has COVID-19 impacted VOC drug development?
The COVID-19 pandemic caused delays in clinical trials and disrupted supply chains but also accelerated the adoption of virtual trials and remote monitoring.